
The “acidification of the ocean”… what does it really mean ? Could the water be like vinegar? What is the impact on the environment? Why does everybody talk about it?
What is acid?
Acids and alkalis are common in daily life. They are found in the home, in our bodies, in industry, car batteries and school science labs.
The term “acid” designs every substance capable of yielding one or more protons (H+). Conversely, an “alkalis” substance can capture one or more protons. Other elements are not acidic, or basic, like water. They are called “neutral”.
Each acid or alkalis is qualified according to the pH scale. It runs from 0 to 14, with 7 being a neutral pH. Anything lower than 7 is acid (and anything higher than 7 is basic).

How do the oceans get acid?
The ocean absorbs about 30% of the carbon dioxide (CO2) that is released in the atmosphere. As levels of atmospheric CO2 increase from human activity such as burning fossil fuels (e.g. car emissions) and changing land use (e.g., deforestation), the amount of carbon dioxide absorbed by the ocean also increases. When CO2 is absorbed by seawater, a series of chemical reactions occur resulting in the increased concentration of hydrogen ions, which raise the water acid intensity.
In the 200-plus years since the industrial revolution began, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to human actions. During this time, the pH of surface ocean waters has fallen by 0.1 pH units.
This might not sound like much, but the pH scale is logarithmic, so this change represents approximately a 30 percent increase in acidity. So, even if when you taste the sea water, you can’t feel anything except salt … it doesn’t mean it didn’t change!
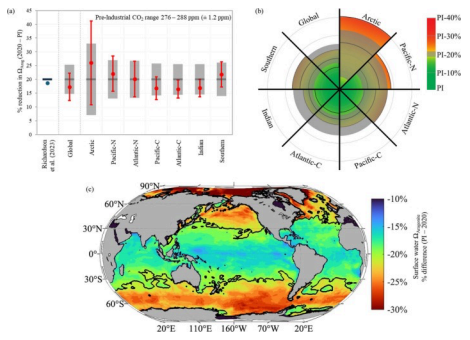
The level of acidification varies partly depending on the region.
The impact on the submarine biodiversity
The ocean acidification has terrible consequences on the biodiversity living there. Indeed, fauna and flora are highly fragile. Corals, oysters, clams, mussels... but also certain species of phytoplankton are under threat.
As a matter of fact, the chemistry of the oceans changes too rapidly for the species to adapt. It confuses fish and their sense of smell, the shells and skeletons of sea creatures dissolve (corals, shellfish…), and causes stress that prevent the species from a correct reproduction.
Of course, this threat also concerns humans, since the fisheries resources are vital for us. For our nourishment, around 80 millions tones of fishes are caught every year!


Planetary Boundary
The planetary boundaries assessment defines nine large scale Earth-system processes and associated boundaries that, if crossed, could generate unacceptable environmental change. These nine processes are:
- climate change
- rate of biodiversity loss (terrestrial and marine)
- interference with the nitrogen and phosphorus cycles
- stratospheric ozone depletion
- ocean acidification
- global freshwater use
- change in land use
- chemical pollution
- atmospheric aerosol loading

Since 2009, six of them have been crossed, proving the efforts of our society to protect the Earth still have to be continued!
What can we do?
REDUCE OUR ATMOSPHERIC POLLUTION!
And how can we do that?
Unfortunately, the big part of the work remains to the authorities and the industry leaders, who have the ability to activate the change on a large scale.
But good news, we also have our responsibility in the protection of our oceans. By adopting a low consumption life-style and avoiding our carbon-intensive activities (taking airplane, using car for every route, heating methods using non-renewable energy sources … we could reduce the atmospheric pollution by 2 tonnes of CO₂ per year and per person.
You can also become part of the change by getting in involved in environmental associations and NGOs like Greek Eco Project. Greek Eco Project.

Written by Deborah Morelle

